In 2013, Bayer Healthcare purchased the permanent contraceptive device Essure from its manufacturer, Conceptus.
About 39,000 persons have filed Essure lawsuits alleging significant adverse health effects. Bayer has settled several of these lawsuits, and the firm has decided not to distribute Essure after 2019.
When will the Essure lawsuit payout be made public? How to join Essure lawsuit? This update on the Essure lawsuit provides details on the current status of complaints lodged against Bayer for complications associated with long-term contraception and information on who is eligible to file a product liability claim and receive compensation.
Essure Lawsuits: How to join Essure lawsuit?
The graph has mostly stayed the same after Bayer settled the first round of Essure litigation. If you or a loved one has an Essure implant, had discomfort or damage, and had surgery to remove the device, you should speak with an experienced attorney to learn the likelihood of moving forward with a lawsuit. Women who got the implants before the FDA issued a black box warning in 2016 are still vulnerable to harm.
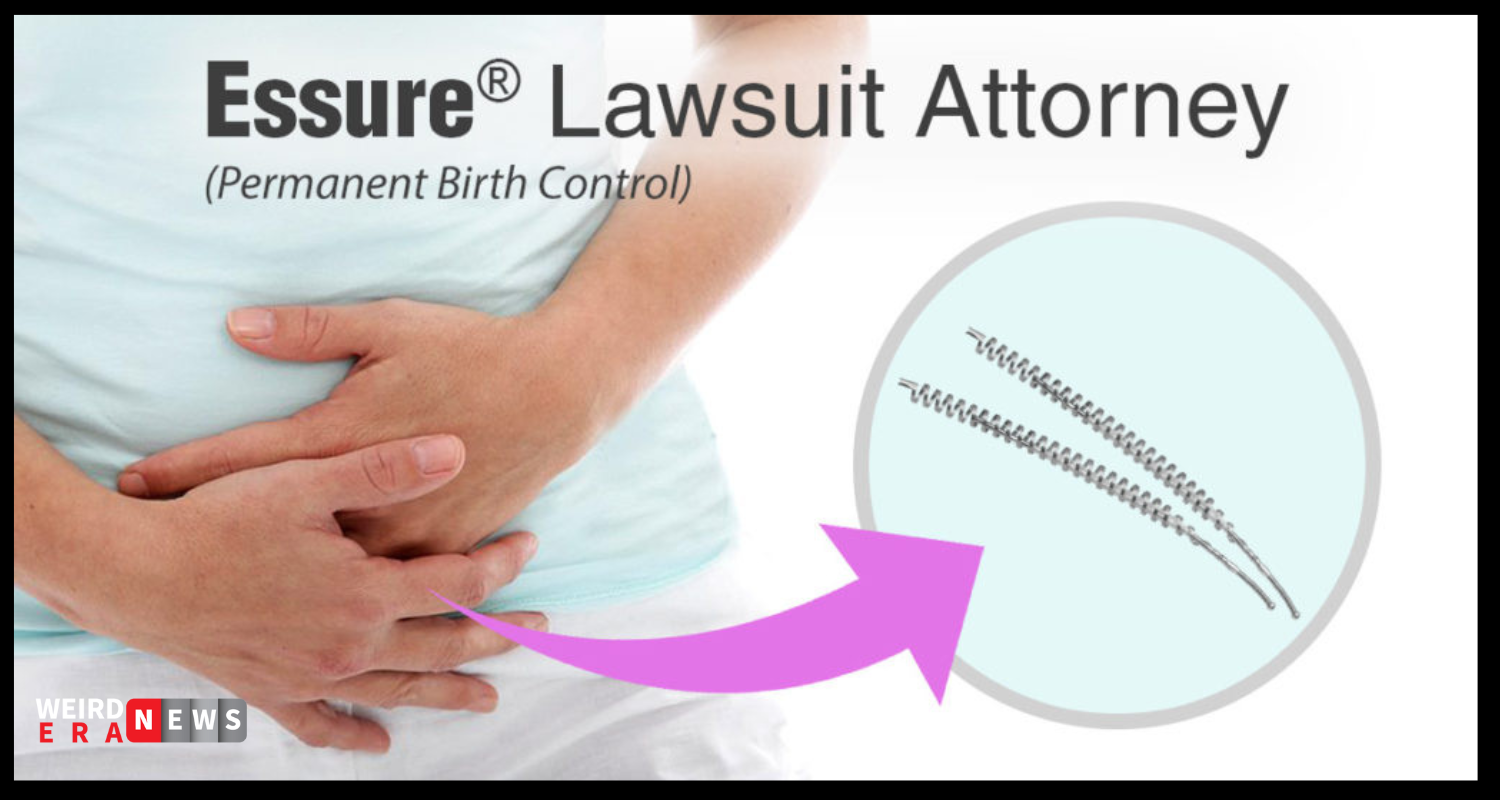
By bypassing the uterus and putting two flexible coils into the fallopian tubes, Essure is a long-term form of contraception.
After a doctor inserts the coils, they should stop fertilized eggs from entering the uterus and keep any more eggs from being fertilized. Unlike tubal ligation, which requires an incision, the Essure device does not, and hence has become a popular non-surgical alternative for over 750,000 women.
Some of them have experienced difficulties. Between 2002 and 2017, the Food and Drug Administration received more than 26,700 reports of adverse events. In response, the FDA requested more clinical trials from Bayer in February 2016 and issued a “black box” warning about the product’s potential adverse effects.
When women experienced complications from Essure, many turned to the legal system to seek compensation for their ordeal. Bayer Pharmaceuticals was named in the lawsuits since it purchased the drug’s original maker, Conceptus, in 2013. According to the complaints against Bayer, the company concealed severe, unintended consequences associated with Essure from both the Food and medicine Administration and the broader public.
![How to join Essure lawsuit?]() How long do I have to start a lawsuit against Essure?
How long do I have to start a lawsuit against Essure?
Each state has its statute of regulations for personal injury claims. Statutes of limitation define these temporal limits. Suppose affected parties need to submit a lawsuit against the manufacturer within the law of the limitation period. In that case, they will be permanently barred from seeking redress from businesses or individuals who may be accountable for their injuries.
The deadline for filing a claim for damages in the event of an injury sustained after the insertion of Essure may have passed or may so soon. We must evaluate your case’s specific before we can provide an answer.
Claims and counterclaims in cases involving Essure
To recover damages in the product liability lawsuit, the plaintiff need not show that the drug maker was careless. Plaintiffs should be able to recover damages under strict liability rules even if the maker was not at fault for the drug’s failure to prevent the claimed side effects when taken as directed.
One of the earliest Essure cases claimed that the business was selling a device that “migrates from the (fallopian) tubes, perforates organs, breaks into pieces, and corrodes, causing havoc on the female body.” True, this would indicate a problem with the product.
Numerous Essure lawsuits, however, claimed that Bayer was negligent in their production of the device. In particular, Bayer was charged with breaking the terms of Essure’s conditional FDA pre-market approval by failing to follow FDA rules and regulations.
- Making Essure devices with illegal components.
- Manufacturing of Essure devices outside of authorized sites.
- Hiding the adverse effects of Essure and not telling the FDA about them.
- Failure to follow Food and Drug Administration regulations in preparing doctors to implant Essure.
- Lackadaisical marketing of Essure to the point of distraction.
- Permitting medical professionals to implant gadgets without proper training Monthly sales of Essure kits to unqualified doctors are encouraged. Substituting a computerized Essure simulator for actual hands-on practice in medical education.
- Seeking medical personnel who need to be adequately trained on the equipment.
Moreover, several claimants claimed that Bayer breached express warranties and asked the court to award damages. This type of claim for breach of contract arises when the warrantor makes representations about the item’s quality that is later proven untrue.
What do I do if I want to go to court?
See a doctor immediately if you’ve been negatively affected by Essure or have experienced one of the significant issues associated with the device. Your doctor and the Food and Drug Administration need to know what happened. you cant get more information about Lawsuit from this link.
What Kinds of Harmful Effects and Injuries Have Been Linked to Essure Birth Control?
Women who have Essure implanted have been reported to experience various problems. Women have reported many issues that they attribute to Essure, including the ones listed below.
- Adhesions.
- Immune system problems.
- Pelvic pain that persists over time.
- Eruption of a coil.
- Pregnancies that start outside of the uterus or are ectopic.
- Balding or shedding of hair.
- Perforations in the intestines.
- Colon or bowel blockage.
- The coil has migrated and is now affecting other organs.
- Migraines.
- Miscarriage.
- Sexual discomfort.
- Scarring.
- Harmful microbes.
- Stillbirths.
- Fetal fluids leak from the uterus.
- Punctures to the uterus.
- Pregnancies that aren’t wanted.
Some women have to have hysterectomies because of the discomfort caused by Essure, and others continue to suffer from agony long after the coil has been removed.
Can I File an Essure Lawsuit?
You may be able to file an Essure case if you or a loved one had the device implanted and then had severe consequences like pain, an unintended pregnancy, ruptured organs, stillbirth, ectopic pregnancy, or the loss of a loved one.
To be eligible to file a lawsuit against Essure, you must be able to demonstrate that you suffered from a medical condition related to long-term birth control. You also need to show that there is a reasonable link between the gadget and health problems.
How long do I have to sue Essure?
In August 2020, Bayer settled most Essure claims for $1.6 billion. It was expected that the settlement would close 90% of the 39,000 pending cases.
If you still need to file a lawsuit and weren’t a part of the settlement, you may still be able to do so.
The deadline before the statute of limitations may vary depending on the type of claim you are making and the location you are claiming in. In many places, the statute of limitations for filing a product liability claim is only two years from the date of the injury. You must complete the deadline to submit your claim in court to retain the right to do so forever.
If you discover the harm was the consequence of dishonest concealment after the fact, the limitation does not apply. Potential plaintiffs may argue that Bayer concealed information about Essure’s harmful side effects by hiding adverse event reports.
Can I join a class action lawsuit for Essure?
There has never been a formal class action lawsuit concerning the Essure device, but that hasn’t stopped many women from filing their claims.
You cannot participate in any ongoing class action lawsuits at this time. Bayer settled the vast majority of the 39,000 pending claims it faced as part of a $1.6 billion settlement in 2020.
There is still time to sue Bayer if the applicable statute of regulations has stayed the same.
What is the timeline for my Essure lawsuit?
Some factors affect how long an Essure lawsuit takes, including whether your case is settled out of court or goes to trial. Your case’s intricacy and the extent of your injuries will increase the time it takes to settle.
A case against Essure’s development schedule
The initial court documents required in an Essure lawsuit are called pleadings. When plaintiffs file a complaint, the defendant has an opportunity to respond.
As a follow-up to the pleadings, the discovery phase involves information exchange between the parties. This fact-finding exercise will allow both parties to review the evidence that the other side will submit to support their case.
The parties discuss the case at a pre-trial conference and try to settle. The case will proceed to trial if a settlement cannot be reached. Each side provides evidence to support its claim in a practice that spans several days or weeks.
If your Essure complaint proceeds to litigation, you will have the opportunity to state your case, and Bayer will have the opportunity to raise defenses. In defense of Essure, Bayer has claimed that the preemption clause protects them from legal responsibility. As a result, Bayer argued that the company couldn’t be held liable because the FDA had already approved Essure.
Settlements and Lawsuits Involving Essure
Litigation can be resolved if the parties involved can achieve a settlement. A trial could also result in a settlement being reached. In a trial setting, liability is determined, and compensation for losses is awarded.
Bayer settled some cases involving Essure by offering consumers a lump sum. Those who participated in the settlement agreement were prohibited from suing the corporation again.
Reaction of the FDA to the Essure Lawsuit
The FDA is investigating Essure’s potential for long-term problems in ensuring women’s safety. As a result, it can be helpful to both patients and doctors. Clinical data from post-market monitoring and ongoing long-term safety data collection from women who have had Essure are used in the FDA’s ongoing review.
The FDA stated that it investigated patient concerns, mandated that Bayer complete its post-market research, issued guidelines on the device, revised the labeling to warn of hazards, and finally limited sales and distribution of Essure before and after it was removed from the market. This action was taken to guarantee that all interested females have full access to and are fully informed about the benefits and hazards associated with Essure.
It has asked that women who have been able to keep their implants in for years continue doing so, with the expectation that data on the consequences over time will be collected. It requests that the women get blood testing to see if the device is causing any immunological reactions and if these reactions are related to the Essure-associated disorders that some patients have reported.
The Legal Responsibility for Essure
In the personal injury trials involving Essure, the law of defective product liability was applied. When someone gets hurt by a product because of a flaw in the way it was made, the design is at fault, or (as appears to be the case with Essure) the manufacturer’s failure to provide adequate warning of the product’s risks, a lawsuit alleging product liability can be launched. The following reasons were given in the case against Essure:
- Essure was made for three years without proper authorization.
- Essure underestimated the risks.
- There was a failure to pay attention to detail when issues emerged.
- There were 16,047 reports of possible device malfunction that were not reported to the FDA.
- Facilitating the resale of Essure devices to medical professionals who have yet to receive proper training.
- Manufacturing of Essure implants began in a non-regulated site in 2005.
- Non-approved components can be found in Essure.
Conclusion
Even though women no longer have to worry about being exposed to Essure, the trauma it caused is still fresh in their thoughts. Women have the right to know all the potential side effects of any implantable medical device before deciding whether or not to get one. Due to Bayer’s recklessness in keeping quiet about the device’s risks, the company bears full responsibility for its harm. How to join Essure lawsuit? Although production had been halted, the producers were still responsible for the consequences of their actions.
Although they resolved the initial batch of Essure claims, they are still threatened by the remaining lawsuits. In 2021, has an Essure case been successful for anyone? The 2021 Essure settlement never occurred.
Still, fair compensation for the Essure litigation is on the horizon. Weirdnewsera that you might not find any other platform which gives you all content about health sports business technology and entertainment.
Frequently Asked Questions (FAQs)
How much does a case review cost?
A free legal evaluation is available to anyone who has suffered because of Essure. We are always available to hear your situation and offer our professional opinion free of charge and without further commitment.
Who can claim Essure problems or sue Bayer for Essure?
A lawsuit can be filed for any woman who experienced pain, bleeding, internal injury, an allergic reaction, or another Essure problem. Also, the loved ones of women who suffered complications from the Essure device may be able to file a claim for damages.
Are some women more susceptible to Essure complications?
There isn’t a specific type of person who is more likely to experience complications from Essure. Complications from having the Essure system implanted are possible for any woman.
How much does it cost to sue Essure for injuries or complications?
No legal fees are due unless we collect for you, and we will provide top-notch representation for anyone filing an Essure lawsuit. If you would like to discuss your legal options for filing an Essure lawsuit with one of our firm’s attorneys, please don’t hesitate to contact us.
Are Essure lawsuits time-limited?
Most states have statutes of limitations on filing a lawsuit against the manufacturer of the birth control device Essure. However, most people who have had the device implanted will still be well within those deadlines if they get in touch with a lawyer soon. Please fill out the form on the right, and one of our attorneys will get in touch with you as soon as possible, usually within the hour, to discuss the deadlines that apply to your claim.

 How long do I have to start a lawsuit against Essure?
How long do I have to start a lawsuit against Essure?