Natural radicals are important intermediates generated at some stage in the redox techniques of organic electrode substances.
But, the terrible stability of organic radicals frequently consequences in the ability to fade natural electrodes, and their stabilization mechanism is hardly ever explored.
Additionally, the structure-property courting between the organic radicals and the ensuing organic electrodes is rarely stated.
Therefore, finely regulating the stability of organic radicals to optimize the electrochemical overall performance of organic electrodes remains a large mission.
Lately, an article titled “Regulating the unconventional intermediates by conjugated devices in covalent natural frameworks for optimized lithium-ion storage” co-authored via Prof.
Zhouguang Lu from SUSTech and Prof. Kaili Zhang from CityU was published in the magazine of energy Chemistry.
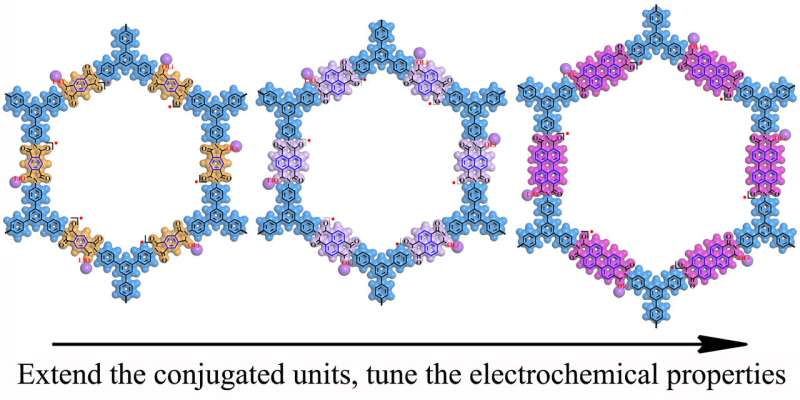
It mentioned a facile and green method to modulate the molecular orbital energies, charge transport capacities and spin electron densities of the lively devices in covalent organic frameworks (COFs) by means of regulating the conjugated unit size to optimize the redox interest and balance of the organic radicals.
COFs based totally on distinct imide conjugated gadgets exhibit tunable discharge voltages, price performance, and biking stabilities.
Particular characterizations and theoretical calculations reveal that imide radicals are important lively intermediates during the redox techniques of those COFs.
Particularly, growing the dimensions of the imide conjugated devices ought to efficiently delocalize the novel electrons and improve the stability of the COFs electrodes.
This observation gives an effective method to modulate the redox chemistry of organic substances for electrochemical strength storage.